Powder Delivery Device
Powder EDS
The powder Exhalation Delivery System (EDS), which is the EDS used in ONZETRA® Xsail® (licensed to Currax Pharmaceuticals LLC), consists of a reusable device body incorporating a flexible mouthpiece to adjust to individual anatomic variations, and a white button piercing assembly to pierce the medication capsule.
Please see Instructions for Use, Important Safety Information, and full Prescribing Information at www.ONZETRAHCP.com.
Disposable nosepieces are provided in a foil pouch to be inserted into the drug delivery device body. Each pre-filled nosepiece section contains a medication capsule containing a dry powder formulation and a clear release tab. The capsule is pierced by pressing and releasing the white button piercing assembly. The flexible mouthpiece and an asymmetrically shaped nosepiece are part of the mechanism that uses the patient’s exhaled breath to naturally seal closed the soft palate and to facilitate delivery of drug to the nasal passages through the sealing nosepiece.
To operate the powder delivery device, a disposable section is inserted into the device body and the piercing button on the side is depressed once. The patient slides the nosepiece of the device into one nostril, then takes a deep breath and blows into the mouthpiece, delivering drug into the nasal passages.
The medication capsule is intended for single-dose administration and is not refillable or removable from the nosepiece. Following drug administration, the disposable nosepiece, including the dose-expended medication capsule, is then removed and discarded.
Watch This Video to See How It Works
 Liquid Delivery Device
Liquid Delivery Device
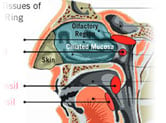 Anatomy of the Nasal Passages
Anatomy of the Nasal Passages
Experience the anatomy of the nasal passages and how it works.
