All your marketing metrics in one place
Visualize your marketing campaigns with concrete metrics that you can pull into any report or presentation

IMAGINE IF A DRUG THAT HAS TRANSFORMED LIVES COULD BE TRANSFORMED TO HELP MILLIONS MORE.
Breathing new life into
proven medicines.
We Are a Company With a Clear Vision
Optinose is a specialty pharmaceutical company focused on creating and bringing to market innovative products for patients with diseases treated by ear, nose, and throat (ENT) and allergy specialists. Our mission is to improve the quality of life for patients. We will accomplish that by becoming the leading specialty pharmaceutical company dedicated to creating innovative products that become standard of care for diseases cared for directly by, or in consultation with, ENT and allergy specialists.
Our first product for these patients uses one of our patented Bi-Directional™ Exhalation Delivery Systems (EDS) to uniquely deliver a potent steroid deep into the nose where inflammation can be hard to reach. Our EDS technology has other applications, such as in treatment of neurological or psychiatric disorders.
Although these areas are not our focus, we have already successfully developed and out-licensed one product in that space, and plan to partner with organizations who focus on central nervous system treatments to develop more.
We Are a Company With a Purpose
The Optinose team is unified by a common shared mission: to improve lives. We will accomplish this while creating value for investors and the healthcare system along with better outcomes for patients and providers. As innovators in every aspect of our work, we will pursue faster and less costly product development, explore evolving commercial business models, and actively seek innovative ways to be more effective and efficient. We are committed to working together as a team that chooses to live every day by core company values that guide our daily behaviors, creating a foundation for future success.
Introducing Exhalation
Delivery Systems
At Optinose, we are breaking through the conventional ways of thinking and doing. We are focused on value creation, starting with our work on a unique and novel drug delivery concept known as Bi-Directional Exhalation Delivery Systems. Our current efforts are centered on developing highly differentiated products for large, underserved markets where EDS technology could redefine the standard of care because it can deliver medication to where it’s needed.
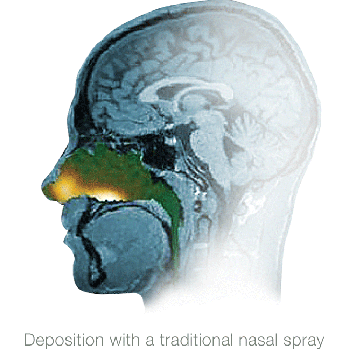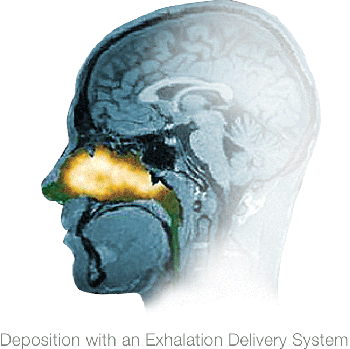
Gamma camera images after using a traditional nasal spray device or an Optinose Exhalation Delivery System. Both images are from the same subject and are representative of the overall findings from 211 images and 56 subjects. The clinical relevance of different deposition patterns has not been established.
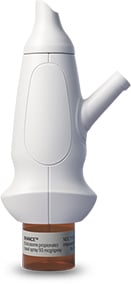 Our Products
Our Products
Our now-approved lead product, XHANCE® (fluticasone propionate), has the potential to become an important treatment option for patients who continue to have unmet needs despite the availability of multiple treatments.
Research & Development
The list of Optinose candidates currently being developed includes:
- XHANCE for chronic sinusitis
We have also identified multiple follow-on pipeline candidates that we believe have outstanding potential to bring new clinical benefits to patients by virtue of our Bi-Directional Exhalation Delivery Systems.
